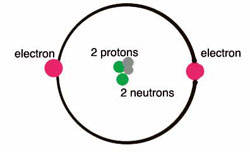
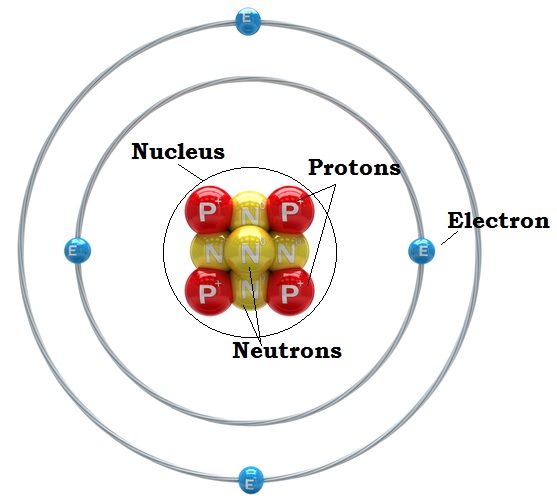
45 parts of a helium atom
Atomic disguise makes helium look like hydrogen 8: Helium Atom - Chemistry LibreTexts - Physical Chemistry The helium atom has two electrons bound to a nucleus with charge Z = 2. The successive removal of the two electrons can be diagrammed as He I1 → He + + e − I2 → He + + + 2e − The first ionization energy I1, the minimum energy required to remove the first electron from helium, is experimentally 24.59 eV.
Helium (He) - Physical & Chemical Properties, Uses, Isotopes - BYJUS Helium falls under inert gas since its outermost electron orbital is full of two electrons. Helium can also be found in lasers, compressed air tanks and coolant in nuclear reactors. It holds the lowest boiling and melting points amongst all other elements. Nuclear fusion of hydrogen in stars generates a significant amount of helium. Isotopes
Parts of a helium atom
BrainPOP BrainPOP ... Loading... ... Which part of a helium atom is positively charged? - Answers Which part of a helium atom is positively charged? Wiki User ∙ 2017-11-21 05:36:54 Study now See answers (2) Best Answer Copy nucleus the nucleus contains protons (positive) and neutrons... Helium - Periodic Table and Atomic Properties Atomic Number - Protons, Electrons and Neutrons in Helium Helium is a chemical element with atomic number 2 which means there are 2 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z.
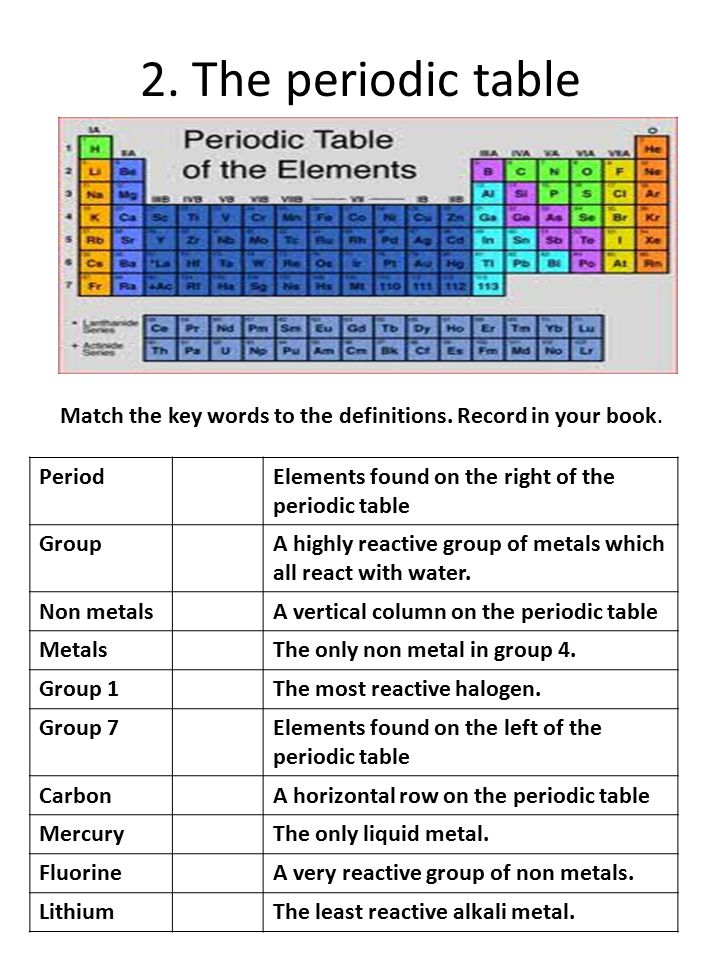
Parts of a helium atom. 8: The Helium Atom - Chemistry LibreTexts The helium atom has two electrons bound to a nucleus with charge Z = 2. The successive removal of the two electrons can be diagrammed as \ [\ce {He}\xrightarrow {\textit {I}_1}\ce {He}^++e^-\xrightarrow {\textit {I}_2}\ce {He}^ {++}+2e^-\label {1}\] 1. Atomic structure Copy and label the parts of the Helium atom. 2. The periodic table Match the key words to the definitions. Record in your book. Period Elements found on the right of the periodic table Group A highly ... Helium atom - Pinterest Helium atom · Helium, the second element of the periodic table, with 2 protons, neutrons and electrons. · More like this. Label the parts of the helium atom pictured below. Atoms. Label the parts of the helium atom pictured below. -proton. EESTIS. 4. O electron. Earth Science IF8755. 1 proton orbit (shell).
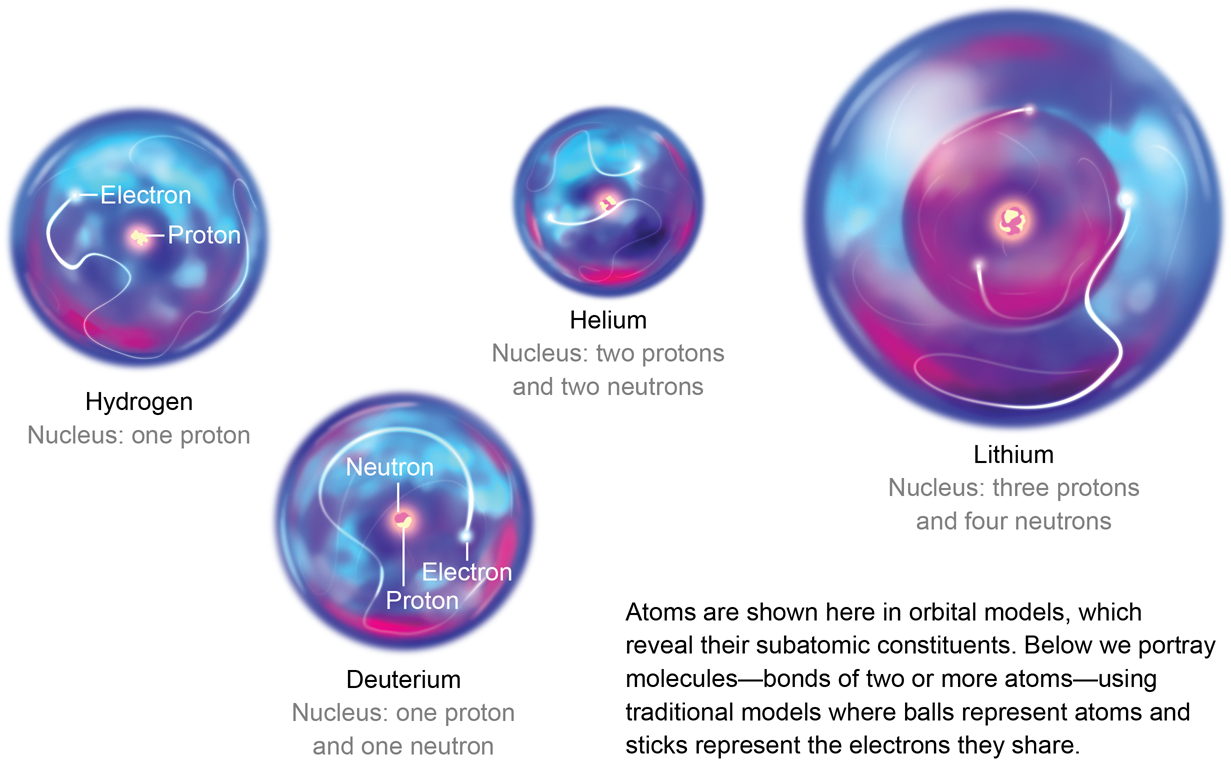
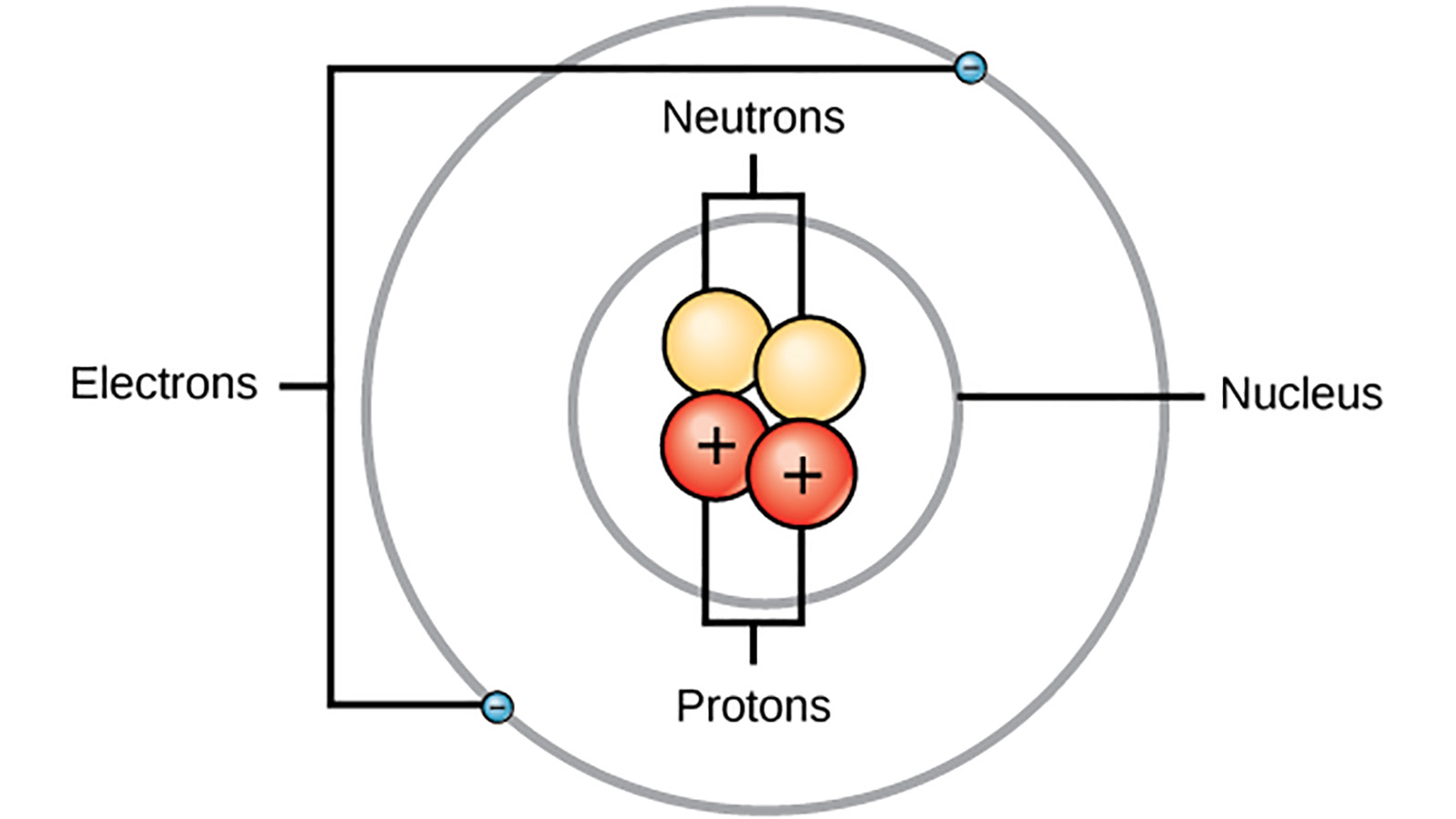
The Structure of the Atom | Astronomy | | Course Hero Figure three is a schematic diagram of a helium atom in its lowest energy state. Two protons are present in the nucleus of all helium atoms. In the most common variety of helium, the nucleus also contains two neutrons, which have nearly the same mass as the proton but carry no charge. Two electrons orbit the nucleus. How to Draw a Helium Atom - Sciencing Apr 24, 2017 ... The nucleus contains uncharged neutrons and positively charged protons, whereas the orbiting electrons possess negative charges. Most helium ... What Are The Parts Of An Atom? - Universe Today Structure Of The Atom: Our current model of the atom can be broken down into three constituents parts - protons, neutron, and electrons. Each of these parts has an associated charge, with... Helium atom - Wikipedia A helium atom is an atom of the chemical element helium. Helium is composed of two electrons bound by the electromagnetic force to a nucleus containing two protons along with either one or two neutrons, depending on the isotope, held together by the strong force.
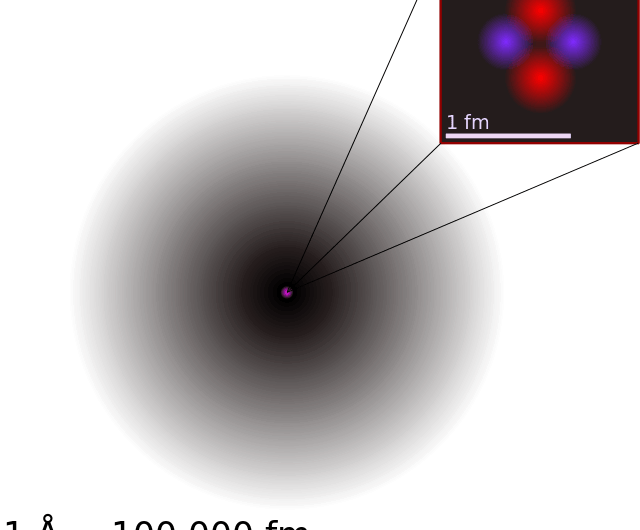
Helium Atom - an overview | ScienceDirect Topics The alpha particle, structurally equivalent to the nucleus of a helium atom and denoted by the Greek letterα, consists of two protons and two neutrons. It is emitted as a decay product of many radionuclides predominantly of atomic number greater than 82. Helium - Wikipedia Helium (from Greek: ἥλιος, romanized: helios, lit. 'sun') is a chemical element with the symbol He and atomic number 2. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas and the first in the noble gas group in the periodic table. Its boiling and melting point are the lowest among all the elements.It is the second lightest and second most abundant element in the ... The Helium Atom - University of California, San Diego The Helium ground state has two electrons in the 1s level . Since the spatial state is symmetric, the spin part of the state must be antisymmetric so (as it always is for closed shells). For our zeroth order energy eigenstates, we will use product states of Hydrogen wavefunctions. and ignore the perturbation. The energy for two electrons in the ... What is the atomic structure of helium? - Quora Well, my knowledge of the atom tells me that if you combine two hydrogen atoms, you will not get a helium atom. Hydrogen has an atomic mass of 1, and helium has ...
Picture Of Helium Atom Pictures, Images and Stock Photos Browse 144 picture of helium atom stock photos and images available, or start a new search to explore more stock photos and images. Green balloons, green and white bubbles beautiful holiday texture, background. Blank for the designer. Pink balloons, pink bubbles beautiful birthday texture.
10 Helium Facts - Atomic Number 2 on the Periodic Table - ThoughtCo 10 Helium Facts. The atomic number of helium is 2, meaning each atom of helium has two protons . The most abundant isotope of the element has 2 neutrons. It is energetically favorable for each helium atom to have 2 electrons, which gives it a stable electron shell. Helium has the lowest melting point and boiling point of the elements, so it ...
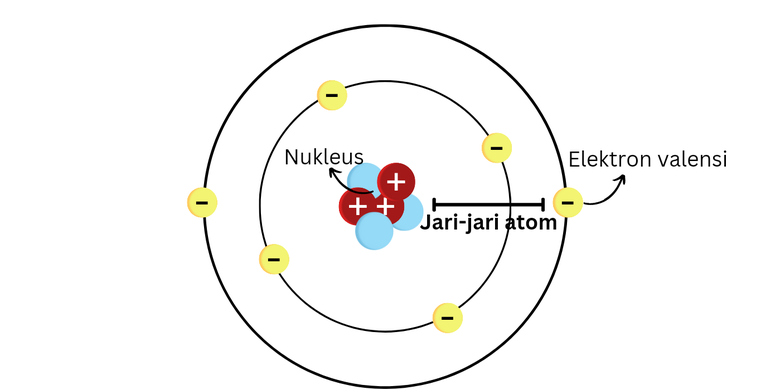
Atom - Wikipedia If an atom has more or fewer electrons than protons, then it has an overall negative or positive charge, respectively – such atoms are called ions. The electrons of an atom are attracted to the protons in an atomic nucleus by the electromagnetic force. The protons and neutrons in the nucleus are attracted to each other by the nuclear force ...
Helium Facts - Atom, Properties, Uses, Gas, Balloons, Voice, Element He Helium is a chemical element with the symbol He and atomic number 2. Helium is a colorless, tasteless and odorless gas. Helium is the second most common element in the Universe (after hydrogen), making up around 24% of its mass. Helium is part of a group of chemical elements called noble gases, the other five that occur naturally are neon ...
What are the parts of an atom? - Phys.org Dec 16, 2015 · Radioactive Decay: Any two atoms that have the same number of protons belong to the same chemical element. But atoms with an equal number of protons can have a different number of neutrons, which ...
Periodic Table – Royal Society of Chemistry Interactive periodic table with element scarcity (SRI), discovery dates, melting and boiling points, group, block and period information.
Helium Atom - an overview | ScienceDirect Topics The nucleus of the helium atom consists of two protons and two neutrons. It is sometimes referred to as the alpha particle and was first observed in the decay of heavy radioactive nuclei.
Browse Articles | Nature Dec 01, 2022 · The authors show that, in a chronic social defeat stress rodent model, a subset of male and female mice avoided social interaction with non-aggressive, same-sex juvenile mice and did not develop ...
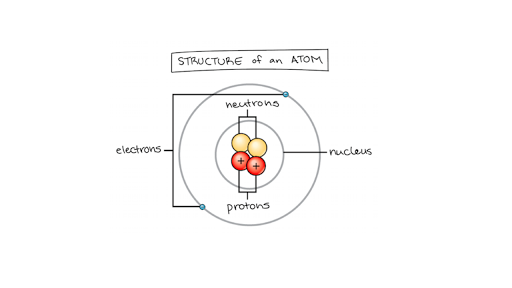
The Structure of an Atom: Parts, Diagram, Examples - Embibe Exams Ans: An atom consists of two parts: the nucleus and extranuclear portions. The nucleus is present in the centre of the atom and is surrounded by the extranuclear portions. Protons and neutrons reside in the nucleus and are together called nucleons. The nucleus is positively charged since the proton is positively charged and the neutron is neutral.
Parts of an Atom: Overview & Structure | What is an Atom? - Video ... They are comprised of three main parts: Protons Neutrons Electrons These are defined at length later in this lesson. This artistic representation of a carbon atom shows protons, neutrons, and...
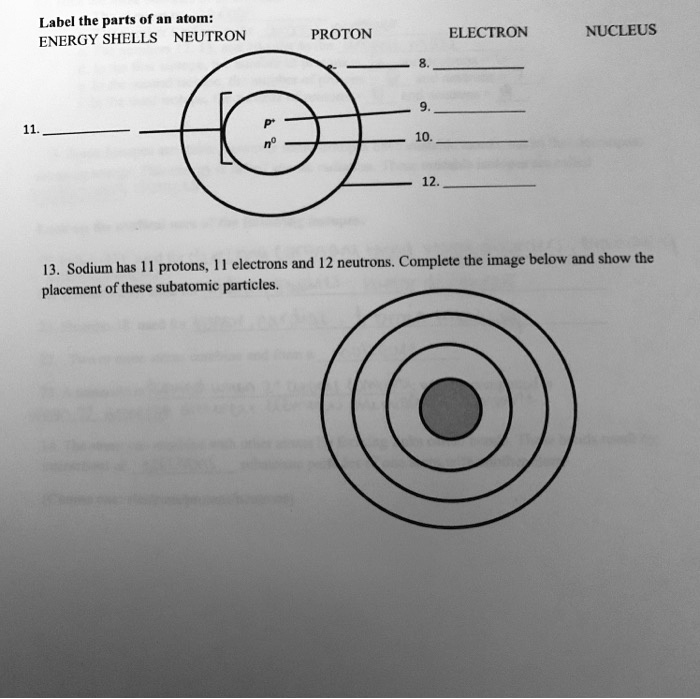
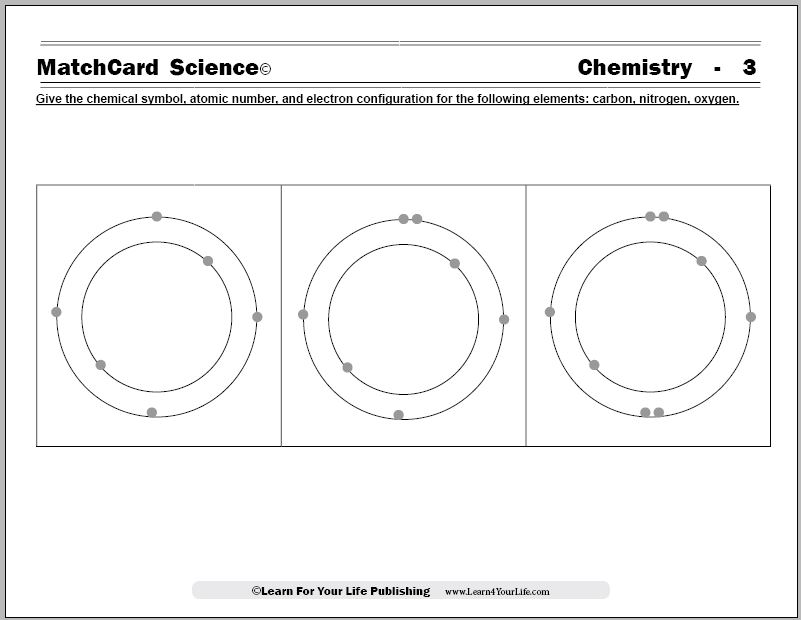
Atomic Structure Quiz Flashcards | Quizlet Terms in this set (9) Draw and Label the parts of a helium atom. Include the mass and charge of each subatomic particle. Should have two protons and two neutrons and 2 electrons and an electron cloud. protons: +, 1 amu neutrons: 0, 1 amu electrons: -1, 1/1840 amu Explain the difference between the atomic number and the mass number of an atom.
Build an Atom - Atoms | Atomic Structure | Isotope Symbols ... Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Then play a game to test your ideas!
Helium Element Properties, Uses, Information, Facts Short Electronic Configuration of Helium atom Short Electronic Configuration of Helium is 1s 2 Block of Helium in Periodic Table There are four blocks (s, p, d, f) in the periodic table. Helium belongs to the s-block of the periodic table. S block is also the first block in the periodic table. Group of Helium element in Periodic Table
What is helium? Atoms, elements, and chemistry - Quatr.us Study ... Aug 7, 2019 ... Helium is a simple atom. The nucleus of a helium atom has two protons and two neutrons. Around the nucleus, there are two electrons.
What is an atom? Facts about the building blocks of matter ... Dec 15, 2021 · Atoms were created after the Big Bang 13.7 billion years ago. As the hot, dense new universe cooled, conditions became suitable for quarks and electrons to form. Quarks came together to form ...
Which part of helium atom is positively charged - Brainly.com 3. Neutrons : These are subatomic particles with no charge located inside the atoms in the nucleus. Protons and neutrons of an atom together makes nucleus of an atom.This is the part where atoms has positive charge. Mass of the atom = P (protons) +N (neutrons ) Atomic number = Number of protons (P) Helium has atomic number of 2.
Helium - Element information, properties and uses | Periodic Table Helium can be found in certain parts of the world, notably in Texas, as a minor component in some sources of natural gas. The interesting thing is how this gas gets into the ground in the first place. ... This alpha-particle is actually just the heart of a helium atom - its nucleus. Once it has grabbed a couple of electrons, a helium atom has ...
Build An Atom - Oak Ridge Institute for Science and Education After this activity, the student will be able to describe the basic structure of matter, name the parts of an atom, have experience using the Periodic Table, explain elements, and have the background to understand isotopes. ... General questions about the properties of elements assume standard temperature and pressure (helium is liquid below ...
Helium | Definition, Properties, Uses, & Facts | Britannica Ordinary air contains about 5 parts per million of helium, and Earth's crust is only about 8 parts per billion. The nucleus of every helium atom contains two protons, but, as is the case with all elements, isotopes of helium exist. The known isotopes of helium contain from one to six neutrons, so their mass numbers range from three to eight.
Matter, elements, and atoms | Chemistry of life (article) - Khan Academy Matter, elements, and atoms. AP.BIO: ENE‑1 (EU) , ENE‑1.A (LO) , ENE‑1.A.2 (EK) Learn about the structure of the atom, and how atoms make up matter. An atom is the smallest unit of matter that retains all of the chemical properties of an element.
Helium | COSMOS - Centre for Astrophysics and Supercomputing Helium is the second element of the periodic table and thus is an atom with two protons in the nucleus. Most Helium atoms have two neutrons in addition to ...
Helium Atom - University of Texas at Austin Helium Atom A helium atom consists of a nucleus of charge surrounded by two electrons. Let us attempt to calculate its ground-state energy. Let the nucleus lie at the origin of our coordinate system, and let the position vectors of the two electrons be and , respectively. The Hamiltonian of the system thus takes the form (1180)
Which part of a helium atom is positively charged? - BRAINLY Which part of a helium atom is positively charged? - 515691. Wingham253 Wingham253 06/27/2015 Chemistry ... Advertisement Advertisement kmatras1 kmatras1 The correct answer is 3. In any atom, the nucleus will be positively charged because the only ions in the nucleus are neutrons, which have a neutral charge, and protons, which have a positive ...
Helium - Atomic Mass - Atomic Weight - He - Periodic Table Mass numbers of typical isotopes of Helium are 3; 4. The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol N. Neutron number plus atomic number equals atomic mass number: N+Z=A.
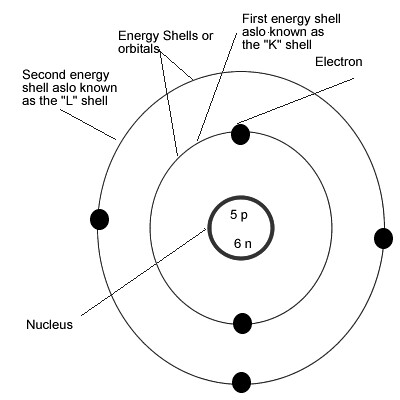
Chem4Kids.com: Helium: Orbital and Bonding Info Helium atomic orbital and chemical bonding information. There are also tutorials on the first thirty-six elements of the periodic table. Check out the blackboard. ... That means there are 2 electrons in a helium atom. Looking at the picture, you can see there are two electrons in shell one. That means the first shell is full.
Helium - Periodic Table and Atomic Properties Atomic Number - Protons, Electrons and Neutrons in Helium Helium is a chemical element with atomic number 2 which means there are 2 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z.
Which part of a helium atom is positively charged? - Answers Which part of a helium atom is positively charged? Wiki User ∙ 2017-11-21 05:36:54 Study now See answers (2) Best Answer Copy nucleus the nucleus contains protons (positive) and neutrons...
BrainPOP BrainPOP ... Loading... ...























Komentar
Posting Komentar