38 what is an open label study
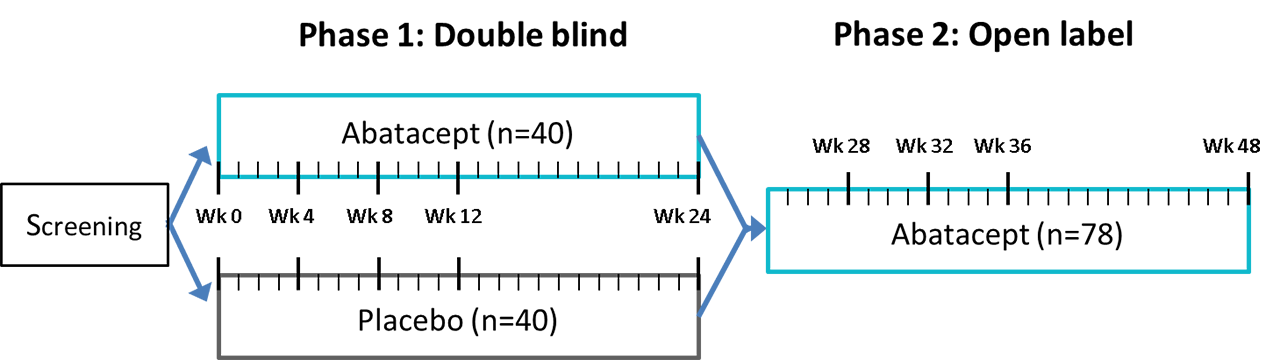
PDF What Are Open-Label Extension Studies For? - The Journal of Rheumatology factors. In the case of open-label extension studies, we feel that analysis of efficacy ought to regard treatment allocation as a possible explanatory variable (in addition to others, such as baseline status and time). The study by Mease and colleagues 1 reports the results of a 48-week open-label extension of a 24-week randomized Effects of open-label placebos in clinical trials: a ... - Nature Open-label placebos (OLPs) are placebos without deception in the sense that patients know that they are receiving a placebo. The objective of our study is to systematically review and analyze...
(PDF) What is an open label trial? - ResearchGate An open label randomised controlled trial study design was used. The control treatment was prazosin alone. The setting was a hospital and research centre in Mahad, a region of India. Participants ...

What is an open label study
open-label trial - Traduction française - Linguee Open-label trial-A clinical trial in which [...] researchers and participants know which drug or vaccine is being administered. · Étude ouverte : un essai ... Spotlight on Open-Label Extension Studies Open-label extension (OLE) studies are common, but they do not receive as much attention as traditional Phase I through Phase IV studies. Enrollment into an OLE study typically follows enrollment into a randomized, blinded, well-controlled main study. › understanding-clinical-trial-terminology-what-is-an-openUnderstanding Clinical Trial Terminology: What is an Open Label... Alternatively, sometimes, trials are conducted in an open-label fashion, meaning study participants and researchers both know which treatment the patient is receiving. Open-label trials can be used to compare treatments or gather additional information about the long-term effects in the intended patient population.
What is an open label study. › understanding-clinical-trial-terminology-what-is-a-longUnderstanding Clinical Trial Terminology: What is a Long-Term... An OLE study is a clinical trial that typically enrolls participants of a previous clinical trial and is designed to gather the long-term safety and tolerability data on a potential new medicine beyond the time period of the main study. Open-label trial - Wikipedia An open-label trial, or open trial, is a type of clinical trial in which information is not withheld from trial participants. [1] In particular, both the researchers and participants know which treatment is being administered. [1] Definition of open label study - NCI Dictionary of Cancer Terms open label study ... A type of study in which both the health providers and the patients are aware of the drug or treatment being given. Search NCI's Dictionary ... What is an open label extension study? • NCK Pharma An open - label trial or open trial is a type of clinical trial in which both the researchers and participants know which treatment is being administered.
Efficacy and safety of lenalidomide in HIV-associated cryptococcal ... And the additional randomized controlled study is required to further validate the finding. Efficacy and safety of lenalidomide in HIV-associated cryptococcal meningitis patients with persistent intracranial inflammation: an open-label, single-arm, prospective interventional study J Neuroinflammation. 2023 Feb 15 ... Evaluation of combination treatment with DS-1205c, an AXL kinase ... An open-label, non-randomized phase 1 study was conducted in Taiwan, in which 13 patients received DS-1205c monotherapy at a dosage of 200, 400, 800, or 1200 mg twice daily for 7 days, followed by combination treatment with DS-1205c (same doses) plus osimertinib 80 mg once daily in 21-day cycles. Treatment continued until disease progression or ... Open-Label Trial - an overview | ScienceDirect Topics An open-label single-dose trial evaluated safety, pharmacokinetics, sensory gating, attention, impulsivity, and inhibition in 12 adult males and females. The single dose of fenobam was associated with a significant improvement of PPI compared to the untreated control group from a previous study ( Berry-Kravis et al., 2009 ). Open label extension studies: research or marketing? - PMC - NCBI Jun 19, 2005 ... Open label extension studies typically follow a double blind randomised placebo controlled trial of a new drug. At the end of the double blind ...
Open-Label Trial - Vial An open-label trial is a clinical trial design where there is no blinding or masking implemented, and investigators and trial subjects are aware of the ... End of Trial and Open-Label Extension (OLE) Frequently Asked Questions ... Open Label Extension, or OLE, is a phase of a study that occurs after the randomized (blinded) portion of the trial is completed if a drug is found to have the potential for benefit. Eligible trial participants take the active form of the drug without placebo. OLE allows active drug to be given to all participants at the same time and to follow them over time. Open label study | definition of open label study by Medical dictionary open-label study a study in which there is no blinding of treatments. Farlex Partner Medical Dictionary © Farlex 2012 open-label study A clinical study in which the patients/subjects and investigators know which product each patient/subject is receiving, which is the opposite of a blinded study. Segen's Medical Dictionary. © 2012 Farlex, Inc. What Is An Open Label Study - November 2022 - Smartstartga.org The purpose of an open label study is to provide information about the experimental treatment to as many people as possible. Open label studies are also used to collect data on the safety and effectiveness of the experimental treatment. Open label studies are sometimes called Phase III studies. This is because Phase III is the final stage of ...
Drug Study Designs | FDA The alternative study design generally proposed to these kinds of studies is an active-treatment concurrent control in which a finding of no difference between the test article and the recognized...
Medical Definition of Open-label - MedicineNet Open-label: A term used to describe the situation when both the researcher and the participant in a research study know the treatment the participant is receiving. Open-label is the opposite of double-blind when neither the researcher nor the participant knows what treatment the participant is receiving. CONTINUE SCROLLING OR CLICK HERE SLIDESHOW
Open-Label Extension Studies | SpringerLink As the name implies, an open-label extension study is an 'appendage' to a randomised controlled clinical trial, usually of an unregistered medicine or intervention. Often the drug is being studied under an investigational new drug (IND) licence or equivalent legislation. The open-label extension study is identified formally as a study.
What is an Open-Label Clinical Trial? - News Medical Open-label trials can be used to gather additional safety and efficacious data on drugs on the market to increase the confidence of clinicians, patients, and clinical bodies. They can play a...
What is an open label trial? | The BMJ An open label randomised controlled trial study design was used. The control treatment was prazosin alone. The setting was a hospital and research centre in Mahad, a region of India. Participants were patients with grade 2 scorpion envenomation, older than 6 months, and with no cardiorespiratory or central nervous system abnormalities.
› media › 87225FDA Guidance: “Design Considerations for Pivotal Clinical ... study design and provide a rationale for the proposed study design. 19. ... – For non-masked (open label) studies this means before any patient reaches an outcome. 36. Closing Remarks
Open-Label Trial - an overview | ScienceDirect Topics Open-label extension studies: In these studies, which often follow a double-blind randomized placebo-controlled trial, subjects have the option of remaining on the study intervention in an open-label fashion (i.e., they know that they are on the study intervention) for an extended period of time (e.g., several years).
clinicalinfo.hiv.gov › en › glossaryOpen-Label Trial | NIH - HIV.gov Open-Label Trial. A type of clinical trial. In open-label trials, both the researchers and participants know which drug (or other intervention) is being given to participants.
medical-dictionary.thefreedictionary.com › open-label+studyOpen-label study | definition of open-label study by Medical... open-label study a study in which there is no blinding of treatments. Farlex Partner Medical Dictionary © Farlex 2012 open-label study A clinical study in which the patients/subjects and investigators know which product each patient/subject is receiving, which is the opposite of a blinded study. Segen's Medical Dictionary. © 2012 Farlex, Inc.
This is a Phase 2, Open-label Study in Which Subjects Seeking Further ... This is a Phase 2, Open-label Study in Which Subjects Seeking Further Improvement of Their SMF Will be Treated With a Second Treatment Session of RZL-012. The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. Listing a study does not mean it has been evaluated by the U.S. Federal Government.
Get the Scoop on an Open-Label Clinical Trial [2023 Guide] An open-label clinical trial is a type of clinical trial in which the volunteers have access to the information about the treatment they are being given. Open-label clinical trials are the opposite of blinded experiments. In blinded experiments, the information is withheld from the participants to avoid bias.
pubmed.ncbi.nlm.nih.gov › 17253876Open-label extension studies: do they provide meaningful ... - ... Open-label extension studies do have a legitimate but limited place in the clinical development of new medicines. The negative perceptions about these studies have arisen because of perversion of acceptable rationales for this type of study and a failure to recognise (or disclose) the limitations resulting from the inherent weaknesses in their ...
A Phase 1/2 Open-label Study to Investigate the Safety Efficacy and ... This study is designed for patients diagnosed with relapsed or refractory B cell precursor acute lymphoblastic leukemia (R/R B-ALL). This study is a 2-part phase 1/2 dose escalation (administration of increasing doses of an investigational drug) and expansion study in adult subjects with R/R B-ALL which will be evaluating the safety, tolerability, pharmacokinetic (PK), and efficacy of ...
seekingalpha.com › article › 4584686-cassava-sciences-open-label-study-dataCassava: Open Label Study Data Unconvincing But Sign Of Good... Mar 5, 2023 · One, this is an open label study, so patients knew what they were taking, drug or placebo. This is severely problematic because Adas-Cog is a very "subjective" test. Patients are given a number of ...
Levels of Evidence - Evidence-Based Medicine - Stony Brook University The American Academy of Family Physicians uses the Strength of Recommendation Taxonomy (SORT) to label key recommendations in clinical review articles. In general, only key recommendations are given a Strength-of-Recommendation grade. Grades are assigned on the basis of the quality and consistency of available evidence. <
› understanding-clinical-trial-terminology-what-is-an-openUnderstanding Clinical Trial Terminology: What is an Open Label... Alternatively, sometimes, trials are conducted in an open-label fashion, meaning study participants and researchers both know which treatment the patient is receiving. Open-label trials can be used to compare treatments or gather additional information about the long-term effects in the intended patient population.
Spotlight on Open-Label Extension Studies Open-label extension (OLE) studies are common, but they do not receive as much attention as traditional Phase I through Phase IV studies. Enrollment into an OLE study typically follows enrollment into a randomized, blinded, well-controlled main study.
open-label trial - Traduction française - Linguee Open-label trial-A clinical trial in which [...] researchers and participants know which drug or vaccine is being administered. · Étude ouverte : un essai ...




























Komentar
Posting Komentar